Challenges in Current Care
Migraine affects an estimated 40 million adults in the U.S.1
40% of patients with migraine reported dissatisfaction with their treatment2,3,*
MIGRAINES
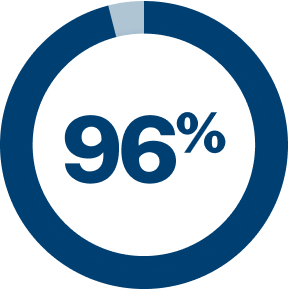
of patients report having at least one unmet need4
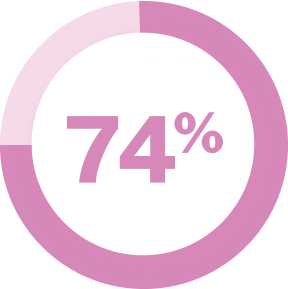
of patients reported their unmet needs were related to inadequate treatment response4
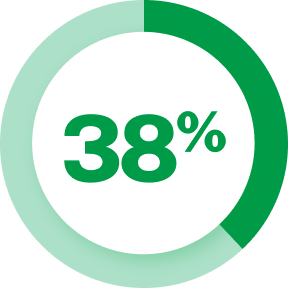
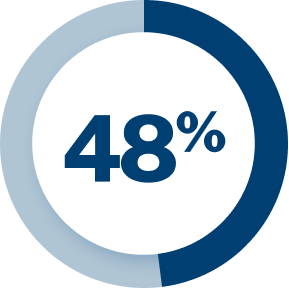
In the U.S. alone, migraine is estimated to account for:

They need something that will work quickly and that’s tolerable for them. And the bottom line is they want to get back to their lives.
-DR. MERLE DIAMOND


Cluster Headache: A painful, underrecognized disorder6
About 1 in 1,000 people suffer from cluster headache and accurate diagnosis of individuals with cluster headache is difficult6,7
Cluster headache may be misdiagnosed as migraine10‑12
KEY DIFFERENCES10-19:
 Cluster Headache | Migraine |
|
|---|---|---|
| Location of Pain | Unilateral10 | Unilateral or bilateral10,13 |
| Type of Pain | Piercing pain with lacrimation10,14 | Throbbing or pulsing pain13-15 |
| Pain Severity | Severe, excruciating pain6 | Moderate-to-severe pain; often varies during the attack16,17 |
| Symptoms |
|
|
| Duration | Can be from 15 minutes to 3 hours10 | Can be from 4 hours to 3 days13 |
| Event
Frequency | From 1 attack every other day to up to 8 per day10 | Typically just a single attack per day17 |
| Patient Presentation | Patients may pace and become agitated or restless10,14,17 | Patients seek rest in a quiet, dark room14,17 |
In the U.S. alone, cluster headache patients had more medical visits compared to patients without cluster headache
Expand
Collapse
WARNING: PERIPHERAL ISCHEMIA FOLLOWING COADMINISTRATION WITH STRONG CYP3A4 INHIBITORS
Serious and/or life-threatening peripheral ischemia has been associated with the coadministration of dihydroergotamine with strong CYP3A4 inhibitors including protease inhibitors and macrolide antibiotics. Because CYP3A4 inhibition elevates the serum levels of dihydroergotamine, the risk for vasospasm leading to cerebral ischemia and/or ischemia of the extremities is increased. Hence, concomitant use of Brekiya autoinjector with strong CYP3A4 inhibitors is contraindicated.
Brekiya autoinjector is contraindicated in patients:
- With concomitant use of strong CYP3A4 inhibitors; with peripheral and central vasoconstrictors; and with other 5-HT1 agonists, ergotamine-containing or ergot-type medications within 24 hours.
- With ischemic heart disease or coronary artery vasospasm.
- With uncontrolled hypertension, peripheral arterial diseases, sepsis, following vascular surgery, or severe hepatic or renal impairment.
- Who have hypersensitivity to dihydroergotamine, ergot alkaloids, latex, or any of the ingredients in Brekiya autoinjector.
- Myocardial Ischemia and/or Infarction, Other Cardiac Adverse Reactions, and Fatalities: In patients with risk factors predictive of coronary artery disease, consider first dose administration under medical supervision with an electrocardiogram.
- Cerebrovascular Adverse Reactions and Fatalities: Cerebrovascular hemorrhage, subarachnoid hemorrhage, and stroke have been reported; discontinue Brekiya autoinjector if suspected.
- Other Vasospasm Related Adverse Reactions: Brekiya autoinjector may cause vasospasm or elevation in blood pressure. Discontinue if signs or symptoms of vasoconstriction develop.
- Medication Overuse Headache: Detoxification may be necessary.
- Preterm Labor: Advise pregnant women of the risk.
- Fibrotic Complications: Pleural and retroperitoneal fibrosis have been reported following prolonged daily use of dihydroergotamine mesylate. Do not exceed the Brekiya autoinjector dosing guidelines or use for chronic daily administration.
Serious cardiac events (including fatal) that have been reported with dihydroergotamine mesylate injection use include: coronary artery vasospasm, transient myocardial ischemia, myocardial infarction, ventricular tachycardia, ventricular fibrillation.
- For subcutaneous injection only.
- Recommended dosage is 1 mg administered subcutaneously as a single 1 mL autoinjector.
- Do not exceed 3 mg (3 doses) in a 24-hour period.
- Do not exceed 6 mg (6 doses) in a week.
- Prior to initiation, a cardiovascular evaluation is recommended.
- Beta Blockers/Nicotine: May potentiate/
provoke vasoconstriction. - Selective Serotonin Reuptake Inhibitors: Weakness, hyperreflexia, and incoordination may occur with coadministration.
- Pregnancy: Based on animal data, may cause fetal harm.
- Lactation: Advise patients not to breastfeed during treatment with Brekiya autoinjector and for 3 days after the last dose.
Brekiya autoinjector is indicated for the acute treatment of migraine with or without aura and the acute treatment of cluster headaches in adults.
Limitations of Use:
Brekiya autoinjector is not indicated for the preventive treatment of migraine or for the management of hemiplegic migraine or migraine with brainstem aura.